
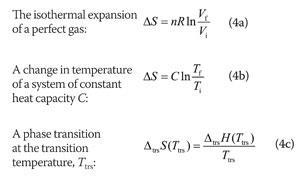
The equation for the change in entropy, Δ S Δ S, isĪbsolute temperature is the temperature measured in Kelvins. The unavailability of energy is important in thermodynamics in fact, the field originated from efforts to convert heat to work, as is done by engines. Consequently, not all energy transferred by heat can be converted into work, and some of it is lost in the form of waste heat-that is, heat that does not go toward doing work. Hence, entropy always tends to increase.Īlthough all forms of energy can be used to do work, it is not possible to use the entire available energy for work. The flow of any energy is always from high to low.
It measures how much energy has been dispersed in a process. Entropy can be thought of as a measure of the dispersal of energy.

When a hot object is placed in the room, it quickly spreads heat energy in all directions. When water in a dish is set on a counter, it eventually evaporates, the individual molecules spreading out in the surrounding air. For instance, if a car tire is punctured, air disperses in all directions. However, we see examples of entropy in our everyday lives. The Relationship between Internal Energy and Entropyīecause the quantity of heat transferred (q rev) is directly proportional to the absolute temperature of an object (T) (q rev ∝ T), the hotter the object, the greater the amount of heat transferred.The meaning of entropy is difficult to grasp, as it may seem like an abstract concept. Reactions can also be both spontaneous and highly endothermic, like the reaction of barium hydroxide with ammonium thiocyanate shown in Figure \(\PageIndex\] Similarly, many salts (such as NH 4NO 3, NaCl, and KBr) dissolve spontaneously in water even though they absorb heat from the surroundings as they dissolve (i.e., ΔH soln > 0). For example, at a pressure of 1 atm, ice melts spontaneously at temperatures greater than 0☌, yet this is an endothermic process because heat is absorbed. But although it is true that many, if not most, spontaneous processes are exothermic, there are also many spontaneous processes that are not exothermic. Initially, many of them focused on enthalpy changes and hypothesized that an exothermic process would always be spontaneous. That is, by itself the magnitude of the heat flow associated with a process does not predict whether the process will occur spontaneously.įor many years, chemists and physicists tried to identify a single measurable quantity that would enable them to predict whether a particular process or reaction would occur spontaneously. Yet we all know that such a process cannot occur: heat always flows from a hot object to a cold one, never in the reverse direction. As long as the same amount of thermal energy was gained by the frying pan and lost by the water, the first law of thermodynamics would be satisfied. Suppose that a hot frying pan in a sink of cold water were to become hotter while the water became cooler. Now consider the same process in reverse. This transfer of heat from a hot object to a cooler one obeys the first law of thermodynamics: energy is conserved. Eventually both objects will reach the same temperature, at a value between the initial temperatures of the two objects. If a hot frying pan that has just been removed from the stove is allowed to come into contact with a cooler object, such as cold water in a sink, heat will flow from the hotter object to the cooler one, in this case usually releasing steam. Let’s consider a familiar example of spontaneous change. This information, however, does not tell us whether a particular process or reaction will occur spontaneously. You also learned previously that the enthalpy change for a chemical reaction can be calculated using tabulated values of enthalpies of formation. Changes in the internal energy (ΔU) are closely related to changes in the enthalpy (ΔH), which is a measure of the heat flow between a system and its surroundings at constant pressure. The first law of thermodynamics governs changes in the state function we have called internal energy (\(U\)).



 0 kommentar(er)
0 kommentar(er)
